News
If you’re benchmarking or experiencing performance problems in your React apps, make sure you’re testing with the minified production build.

Fibralign receives ISO 13485:2012 Certification
Fibralign received Certification Notification on February 18, 2018 from notifying body DEKRA Certification B. V. that it's management system meets the requirements of EN ISO 13486:2016:2012 + AC:2012. The scope of the certification is for the design, development,...

AngelMD Closes Syndicate Funding Round in Fibralign
AngelMD,an investment and networking platform connecting innovative medical startups, physicians, investors, and industry partners, today announced that it has completed a Syndicate funding round for Fibralign, Inc. Fibralign produces therapeutic medical devices that...

Fibralign Corporation Appoints Dimitris Dionysiou, Ph.D., M.D. as Chief Medical Officer
Fibralign Corporation, a developer of advanced therapeutic medical devices, announces the appointment of Dimitris Dionysiou, Ph.D., M.D. as its Chief Medical Officer. Dr. Dionysiou joins the Company during the clinical phase for its first product, the BioBridge®...
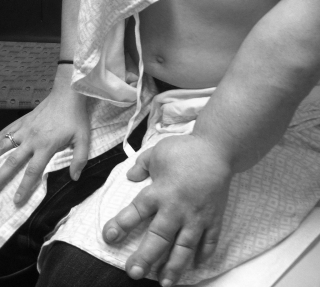
Study shows nanofiber scaffolds could treat lymphedema by rerouting lymphatic system around blockage
Researchers at the School of Medicine have developed a possible treatment for lymphedema, the severe swelling of an arm or leg that can occur when the lymph system is blocked. Using scaffolding composed of specially patterned collagen nanofibers, the researchers...

Fibralign wins 2016 BSK biotech startup competition!
Wins $30,000 first place prize among >150 amazing startups in CA event held April 7th at Stanford Medical school. Sponsored by BioSciKin, a Chinese biotech venture focused on precision medicine. See full article at http://califesciences.org/bioscikin-1/. Fibralign...

BioBridge® Collagen Matrix receives 510(k) clearance
Fibralign's first product, the BioBridge® Collagen Matrix has received 510(k) clearance from the FDA on January 07, 2016 for use in soft tissue support/repair (K151083). Click to view. BioBridge is a sterile thread-like surgical mesh designed for use in surgery to...

Vol. 28 No.1 – 2014 Best New Investigator Award: Development Update: A Promising Surgical Procedure
Michael Paukshto, PhD, CTO and co-founder of Fibralign Corporation, was awarded the 2014 Best New Investigator Award at the 11th Biennial NLN International Conference. Fibralign has developed and established an end-to-end manufacturing system for production of its...

Fibralign wins Medtech Innovator Award
Fibralign won the prestigious Medtech Innovator award at the 2014 WSGRC Medical Device Conference, placing first out of over 300 startups for this top honor and $125,000 prize.
